Interdisciplinary Pharmacology Scientists (TIPS)
(Funded by the National Institutes of Health grant T32GM120011 to The University of Texas Health Science Center at Houston)
The next Call for Predoctoral Applications will be released in Summer 2020.
Program Director:
Carmen Dessauer, PhD, Professor
Integrative Biology and Pharmacology
The University of Texas Health Science Center at Houston
Program Co-Director:
Timothy Palzkill, PhD, Professor and Chair
Pharmacology, Baylor College of Medicine
Program Administrator:
Vanessa C. Herrera, Assistant Director
Keck Center Training, Gulf Coast Consortia
The mission of the Training Interdisciplinary Pharmacology Scientists (TIPS) program is to provide interdisciplinary training in pharmacological sciences to PhD students from a variety of backgrounds to ultimately decrease the time and expense currently required to bring safe, efficacious drugs to clinical application. This training will emphasize the science and technology that underlie drug discovery research and drug development and prepare graduates for careers in academia, the private sector including pharmaceutical and biotechnology industries, and government regulatory agencies. Interdisciplinary training for this program includes screening, computation, medicinal chemistry, structural biology, target discovery and validation, as well as opportunities to explore how to maximize therapeutic benefit, minimize toxicity, implement precision medicine and improve human health.
The multi-institutional TIPS program will leverage the tremendous collaborative environment, research expertise and training experience of 26 interactive faculty from three participating institutions focused on graduate biomedical science education – The University of Texas Health Science Center at Houston, Baylor College of Medicine, and Rice University.
Trainees will benefit not only from traditional experiences, knowledge, and established approaches but also from exposure to recent advances in and systems biology approaches to target validation, drug specificity, safety, human heterogeneity, human trial design, data capture, and interpretation. TIPS is designed to immerse trainees in an array of environments in which they experience the inventiveness and laboratory training historically found in academia, the drug development expertise of biotech and pharma, and regulatory challenges and initiatives of agencies such as the FDA.
Trainee Eligibility
PhD students from The University of Texas Health Science Center at Houston, Baylor College of Medicine and Rice University are eligible to apply.
- Students must be US citizens or Permanent Residents.
- Students may apply after joining a lab near the beginning of their second or third year of graduate school.
- Students must select a Primary mentor from the TIPS Training Faculty.
The Gulf Coast Consortia is committed to providing equal opportunity in training for individuals with disabilities and individuals from racial and ethnic groups who are currently under-represented in STEM fields. We welcome applications from all qualified trainees, regardless of ethnic/racial status or disability status. All GCC member institutions are ADAAA compliant and have offices of disability support services that provide accommodations and support services to trainees, faculty, staff, and visitors.
Current Trainees
Training Interdisciplinary Pharmacology Scientists Application
IMPORTANT: Please Review Trainee Eligibility Criteria above prior to completing an application.
- Application deadline: Monday, September 30, 2019
- Applicant Interviews: October 2019 (Date TBA)
- Fellowship Start Date: November 1, 2019
TIPS APPLICATION INSTRUCTIONS (NOTE: All application material must be received on or before September 30, 2019 for eligible consideration. Late submissions will not be accepted.)
Complete and submit the TIPS Training Application form via email to Vanessa Herrera at herrera@rice.edu. Be certain to fill out the application COMPLETELY: you must provide GRE and/or MCAT scores as well as undergraduate and graduate GPA.
Complete and submit the Project Information document (word or pdf format) via email to Vanessa Herrera at herrera@rice.edu, and include the following sections:
- Project Description (max 750 words)
- Layperson’s Project Description (max 250 words)
- Mentoring Plan (max 500 words)
- Grant Support *Please see full list of detailed requirements for this document in the TIPS Training Application form
The Primary Mentor and Co-Mentor must each submit a Mentor Recommendation Letter via direct email to Vanessa Herrera at herrera@rice.edu. These letters should evaluate the applicant in terms of his/her suitability for a research career in the pharmacological sciences and should specifically address the following areas:
- Scientific background and prior training
- Research experience and skills
- Quantitative skills, aptitude and training
- Work ethic
- Communication skills
- Ability to work with others
- Mentor’s approval of applicant’s Mentoring Plan
Secure two Letters of Recommendation from people other than your mentors. Recommenders should submit their letters directly to Vanessa Herrera at herrera@rice.edu
Submit Transcripts from all undergraduate and graduate coursework (Official preferred, Unofficial accepted) to Vanessa Herrera at herrera@rice.edu
Submit a current resume (CV) outlining your professional work and research experience and your history to Vanessa Herrera at herrera@rice.edu
Submit proof of citizenship – copy of passport, permanent residency card, or birth certificate to Vanessa Herrera at herrera@rice.edu
SELECTION PROCESS (Interview) Selected applicants will be invited to interview with the TIPS Steering Committee. The interview will:
- Be attended by applicant and mentors (both primary and co-mentors).
- Consist of a 10-minute research talk by the applicant with slides of his/her research experience, goals, and proposed collaborative research project.
- Take place in UT Health’s McGovern Medical School (Date, TBA).
This interview is critical to robustly assess the applicant’s suitability for the Program and potential for success during and after the Program, including the ability to communicate his/her science. The Steering Committee will discuss each applicant and make final selections based on merit. All applicants will be notified via email.
APPOINTMENT INFORMATION (For fiscal year 2019-2020)
- Appointments will begin November 1, 2019
- Appointments will be for 1 year, with potential for a 2nd year. The 2nd year appointment is dependent upon 1) successful completion of program requirements in year 1, and 2) continued NIH funding.
- Trainees and mentors will participate in an annual progress review with the TIPS Steering Committee near the end of the 1st year. If trainee progress is acceptable, the trainee could receive a 2nd year of support. Trainees and mentors are also expected to participate in an interview at the end of their training period.
- Stipend level: $24,816
- Travel: $300 (Required poster or oral presentation at selected conference)
- Other funds: Some funds will be provided to help defray costs of trainee Tuition and Health Insurance
PROGRAM REQUIREMENTS Applicants will be required to fulfill the requirements listed below:
- Required courses in therapeutics and drug development (See ‘Curriculum’ tab for details).
- Co-mentored research project to ensure cross-training.
- Career development to expand professional skills and knowledge of career options.
- Broader scientific activities, such as weekly seminars, monthly trainee meetings and an annual research conference, to build a well-rounded, collegial community within and between training cohorts.
- Shadowships at local biotech companies to provide a unique industry experience and facilitate potential collaborations for our trainees.
- Acceptable progress after year 1, as determined by annual progress review with TIPS Steering Committee Trainees are strongly encouraged to participate in Program activities after their training period ends, as their experience and knowledge will be extremely important for future trainees. The Program as a whole strives to build a large cohort of trainees who have received exceptional training in the Pharmacological Sciences.
Trainees should acknowledge NIH/NIGMS’ full or partial support of your research in all publications, such as abstracts, journal articles, oral or poster presentations, news releases, and other communications. Please refer to the Acknowledging Keck Center support page for specific instructions.
The Gulf Coast Consortia is committed to providing equal opportunity in training for individuals with disabilities and individuals from racial and ethnic groups who are currently under-represented in STEM fields. We welcome applications from all qualified trainees, regardless of ethnic/racial status or disability status. All GCC member institutions are ADAAA compliant and have offices of disability support services that provide accommodations and support services to trainees, faculty, staff, and visitors.
|
Dr. Carmen Dessauer, Program Director |
Dr. Timothy Palzkill, Co-Program Director |
|
Dr. Xiaodong Cheng |
Dr. John Hancock |
|
Dr. Lydia Kavraki |
Dr. Natasha Kirienko |
|
Dr. Thomas Westbrook |
|
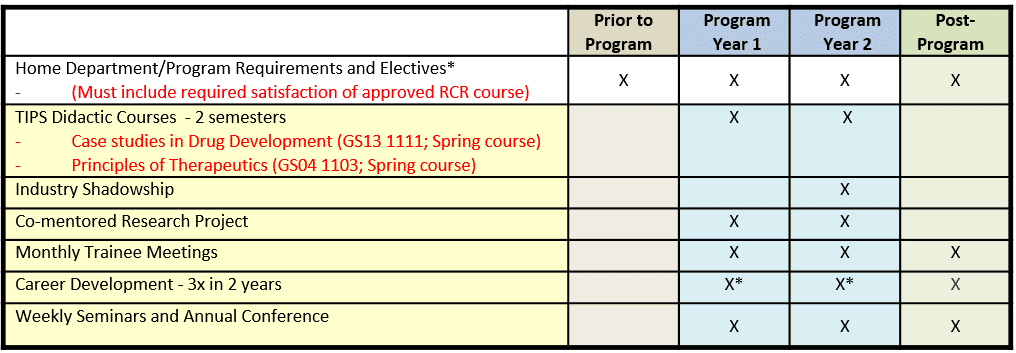
Training Interdisciplinary Pharmacology Scientists Curriculum
The requirements of a TIPS trainee are:
- Complete Case Studies in Drug Development (GS13 1111, 2 credit hours); year 1 of appointment (required).
- Complete Principles of Therapeutics (GS04 1103, 3 credit hours) or equivalent course with prior approval from Program Director; year 1 or year 2 of appointment.
- Show completion of an approved course in the Responsible Conduct of Research (research ethics).
- Enroll every semester in the Rice seminar course BIOC 592 (Friday afternoon Keck Seminar Series); years 1 and 2.
- Present a poster at the Keck Center Annual Fall Research Conference (held annually in the Fall). Years 1 and 2.
- Participate in monthly trainee meetings; years 1 and 2; highly recommended for alums of this Program.
- Participate in a minimum of three career development seminars, workshops or classes; years 1 and 2.
- Complete up-to one week industry shadowships at local biotechnology companies; year 2 of appointment.
Curriculum Model
Click here for link to Inter-Institutional Graduate Course Registration Forms
Click here for link to The University of Texas Health Science Center’s Graduate School
Click here for link to Baylor College of Medicine’s Graduate School web page
Click here for link to Rice University’s Registrar Office
Responsible Conduct of Research (RCR) requirement:
As the TIPS training program is funded by the National Institutes of Health (NIH), per NIH regulation NOT-OD-10-019 all trainees must take an approved Responsible Conduct of Research course at the graduate school level. Even if the trainee has taken an ethics course while an undergraduate, or an online course such as from CITI, the trainee must still take a course at the graduate level (online courses by themselves do not fulfill NIH requirements). Trainees must provide the GCC with a transcript or certificate as proof of completion. Predoctoral students entering their fifth year of graduate school are required to complete a refresher RCR course and provide documentation of completion.
The following are approved Responsible Conduct of Research courses:
- Baylor College of Medicine: Grad 513 Science as a Profession
- The University of Texas Health Science Center at Houston: GS1 10051 The Ethical Dimensions of the Biomedical Sciences
- Rice University: UNIV 594 Training in the Responsible Conduct of Research; formerly BIOS/BIOE 594